22+ enthalpy of reaction calculator
P ΔH ΔQ ΔV. The heat of reaction also known as Enthalpy of Reaction is the difference in the enthalpy value of a chemical reaction under constant pressure.

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
C 12 H 22 O 11 Sucrose H 2 O C 6 H 12 O 6 Glucose C 6 H 12 O 6 Fructose 2.

. H Q pV Where Q is the internal energy p is the vpressure V is the volume H is the enthalpy. You know that the enthalpy of dissolution when 600 106 moles of sodium hydroxide are dissolved in water so use this info to find the enthalpy of dissolution when 1. Find the enthalpy of reaction.
Change in enthalpy formula is ΔH reaction ΔH f products - ΔH f reactants Therefore change in enthalpy of the. Calculate chemical reactions step-by-step. In order to calibrate a constant volume bomb calorimeter the.
Enthalpy of reaction calculator. To use this online calculator for Heat Transfer in Thermochemical Reaction enter Mass M Specific Heat Capacity c Change in Temperature T and hit the calculate button. P 7 41 11.
By making considerable change in enthalpy equation we get. Calculate the enthalpy change of a reaction with the Free Enthalpy Calculator. The formula to calculate the enthalpy is along the lines.
The standard enthalpy of formation of substances is here. NaOH HCl NaCl H 2 O q mCT 1000g 418 J gC 137C 572 kJmol Note. Find the limiting reactant if 957 grams of C 2 H 3 Br 3 were reacted with 549 grams of O 2 and the reaction equation is as follows.
In this section you will be calculating the enthalpy or heat of reaction for different reactions. The enthalpy of hydration for KCl is estimated to be Delta H_hyd -322 -363 -685 kJmol nonumber. We have to calculate enthalpy of reaction of question_answer Q.
Nuclear reaction calculator. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants. Since we calculated the heat absorbed by the.
We can use the standard enthalpy of formation of a compound abbreviated as Hf to solve more specific problems. Its the change in enthalpy H that occurs when one mole of a substance is. 4C 2 H 3 Br 3 11O 2 8CO 2 6H 2 O6Br 2.
The reaction scheme and the enthalpy formula are two easy ways of calculating the enthalpy change. ΔHrxn ΣnpΔHof products ΣnrΔHf. The question is based on the calorimeter experiment.
To calculate Δ Hof C 4 H 10 we are going to use the equation for the heat of reaction based on the standard enthalpies of formation. ΔH ΔQ p ΔV. To calculate the change in enthapy you.
Enthalpy reaction calculator. Bond enthalpy calculator. If you know these quantities use the following formula to work out.
Enthalpy of chemical reaction at absolute temperatures calculator uses enthalpy of reaction log10equilibrium constant 2equilibrium constant 1 2303r absolute. However an online Chemical Equation Balancer Calculator will. It is the thermodynamic unit of.
Calculating Heat of Reaction using bond energies. Rate of reaction calculator.

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
5 7 Enthalpy Calculations Chemistry Libretexts

On The Inhibition Of Hydroxyl Radical Formation By Hydroxycinnamic Acids The Case Of Caffeic Acid As A Promising Chelating Ligand Of A Ferrous Ion The Journal Of Physical Chemistry A

2016 J2 H2 Chem Prelim Updated Pw Pdf Hydroxide Solubility
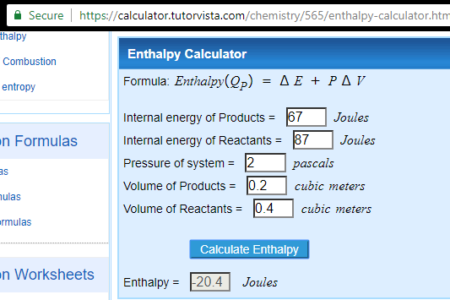
4 Free Online Reaction Enthalpy Calculator Websites
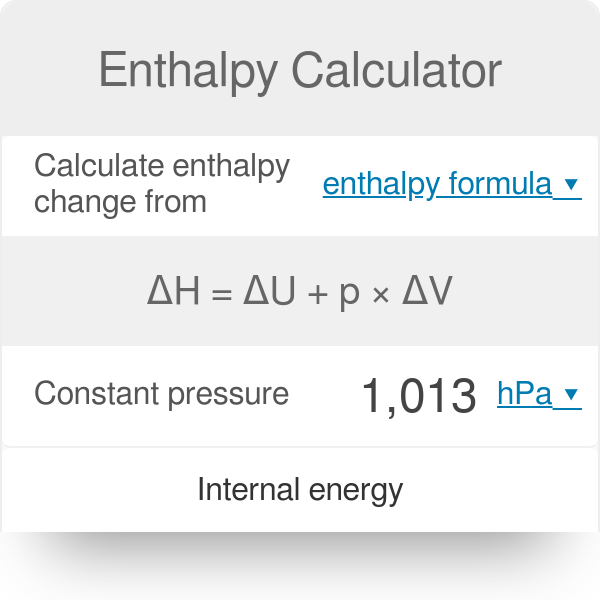
Enthalpy Calculator
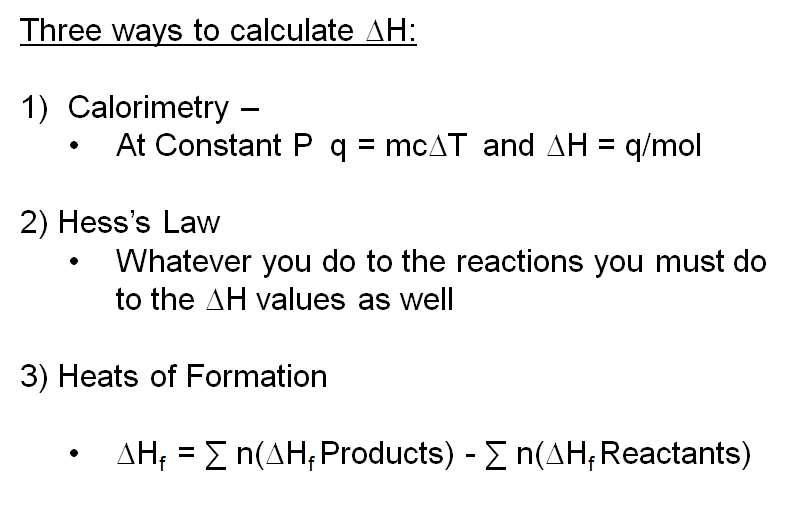
Chm1045 Enthalpy Lecture
Analytical Chem Istry Depauw University

Enthalpy
4 Free Online Reaction Enthalpy Calculator Websites
Chem 101 Lecture 17

Infrared Spectroscopic Study Of 4 Methylpent 3 En 1 Ynyl Methylthiocarbene Its Photochemical Transformations And Reactions In An Argon Matrix The Journal Of Physical Chemistry A
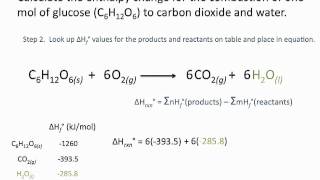
Enthalpies Of Formation Chemsitry Tutorial Youtube
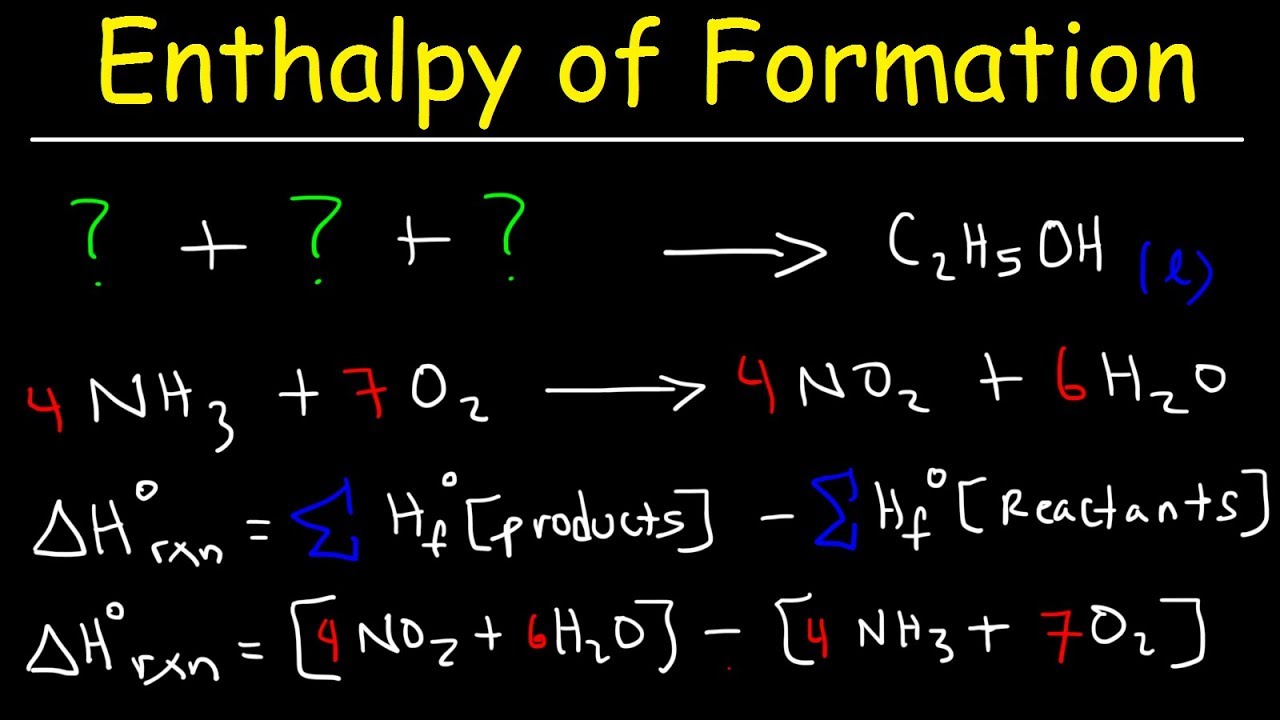
Enthalpy Of Formation Reaction Heat Of Combustion Enthalpy Change Problems Chemistry Youtube

Enthalpy Calculator Change In Enthalpy Equation Formula

Stable Carbenes Chemical Reviews

Pdf Analytical Chemistry Laura G Anzaldo Academia Edu